Adenosine Triphosphate

ATP provides the energy for both energy-consuming endergonic reactions and energy-releasing exergonic reactions, which require a small input of activation energy. When the chemical bonds within ATP are broken, energy is released and can be harnessed for cellular work. The more bonds in a molecule, the more potential energy it contains.
- λ Values at different nucleotide-bound states; the error bars represent the SD among different samples and repeats.
- The DEER data were acquired with chirp pump pulses (74⇓–76, 78) (for set-up see Methods and SI Appendix, Methods and Fig. S7D).
- The gray lines indicate the fit to the data after removing the background signal using the DeerAnalysis software (primary data and validation of the background for the individual DEER traces are in SI Appendix, Figs. S20–S22).
- Mn–Mn DEER time traces at different states of the ATP cycle in WT yHsp90.
- The sample names indicated on the figure are summarized in SI Appendix, Table S1.
- Average Mn–Mn distance distributions obtained upon multiple repeats of sample preparation and/or measurements with the number of different sample preparations indicated (×n).
With the exception of FRET studies where AMP–PNP shifted completely the equilibrium toward the closed conformation , the literature suggests that Hsp90 is found in the nucleotide-bound states in 2 distinct open and closed conformations. The question that arises is why we do not observe the open conformation? This can explain 1) why we observe only the closed states and 2) why the λ values of the Mn–Mn DEER were found low for the pre- and posthydrolysis states . The observation of dimerized C-domains in apo- and nucleotide-bound yHsp90 is in agreement with biochemical studies on human (Hsp90α and Hsp90β) homologs . Additionally, the C-domains were found dimerized in the closed AMP–PNP-bound yHsp90 X-ray crystal structure in the presence of cochaperone p23/Sba1 . Because DEER in frozen solutions reports a superposition of all conformations this difference of 2 nm between the 2 conformations should have been resolved in the Gd–Gd DEER measurement, considering the width of the Gd–Gd distance distribution.
It was reported that Hsp90 adopts more than 1 conformation when nucleotides are present. Specifically, EM showed that in the presence of saturating amounts of AMP–PNP and ADP, the bacterial homolog HtpG is in a mixture of open/closed and open/compact conformations, respectively .
Biological Sciences
Finally, we discuss the DEER results obtained with the spin labels for the N- and M- domains in light of the above discussion. For example, mutant A152C, which is found in the vicinity of the ATPase site (Fig. 1A and SI Appendix, Fig. S11), yielded 2 major populations, one of which (∼3.6 nm) matches well the range of Mn–Mn distances, in addition to a second population with a very broad distribution. The distance distribution centered around ∼3.6 nm can be assigned to the closed conformations of the chaperone based on the X-ray closed structure and our Mn DEER data .
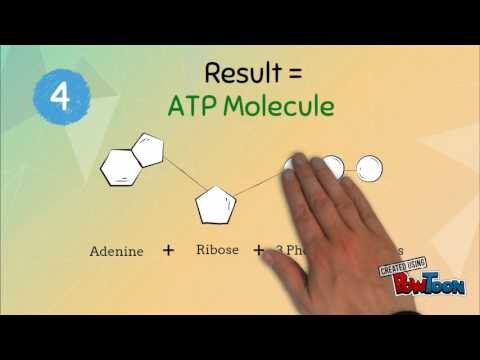
λ Values at different nucleotide-bound states; the error bars represent the SD among different samples and repeats. Adenosine triphosphate is comprised of the molecule adenosine bound to three phosphate groups. Adenosine is a nucleoside consisting of the nitrogenous base adenine and the five-carbon sugar ribose. The three phosphate groups, in order of closest to furthest from the ribose sugar, are labeled alpha, beta, and gamma. The two bonds between the phosphates are equal high-energy bonds that, when broken, release sufficient energy to power a variety of cellular reactions and processes. The bond between the beta and gamma phosphate is considered “high-energy” because when the bond breaks, the products [adenosine diphosphate and one inorganic phosphate group ] have a lower free energy than the reactants .
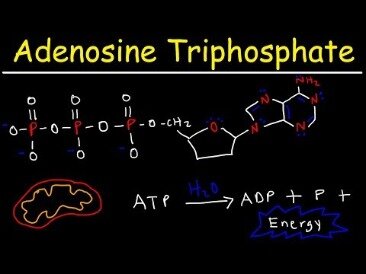
ATP breakdown into ADP and Pi is called hydrolysis because it consumes a water molecule (hydro-, meaning “water”, and lysis, meaning “separation”). ATP is an unstable molecule in unbuffered water, in which it hydrolyses to ADP and phosphate. This is because the strength of the bonds between the phosphate groups in ATP is less than the strength of the hydrogen bonds , between its products , and water. Thus, if ATP and ADP are in chemical equilibrium in water, almost all of the ATP will eventually be converted to ADP. A system that is far from equilibrium contains Gibbs free energy, and is capable of doing work. Living cells maintain the ratio of ATP to ADP at a point ten orders of magnitude from equilibrium, with ATP concentrations fivefold higher than the concentration of ADP.
Are cofactors and coenzymes the same?
Coenzymes are small, non-protein organic molecules that carry chemical groups between enzymes (e.g. NAD and FAD). Forms easily removed loose bonds. Cofactor is a non-protein chemical compound that tightly and loosely binds with an enzyme or other protein molecules.
Hsp90 is found in almost all cell compartments and is one of the most abundant proteins in the cytosol, where it constitutes 1 to 2% of the proteome (1⇓⇓⇓–5). Inhibition of Hsp90’s activity by drugs has been found to abolish these processes (7⇓–9) and, therefore, it is considered as a target for the therapy of cancer and of neurodegenerative diseases (3, 6, 10⇓–12). Hsp90 is a homo-dimer and works off-equilibrium utilizing energy from ATP hydrolysis, where Mg ion is an essential cofactor. Each protomer binds one ATP molecule (13⇓–15) and the ATP hydrolysis rate is slow, ∼0.1 to 1.0 ATP molecule per minute (15⇓⇓⇓–19). Adenosine triphosphate is the energy currency for cellular processes.
Sciencing_icons_environment Environment
The gray lines indicate the fit to the data after removing the background signal using the DeerAnalysis software (primary data and validation of the background for the individual DEER traces are in SI Appendix, Figs. S20–S22). The DEER data were acquired with chirp pump pulses (74⇓–76, 78) (for set-up see Methods and SI Appendix, Methods and Fig. S7D). Average Mn–Mn distance distributions obtained upon multiple repeats of sample preparation and/or measurements with the number of different sample preparations indicated (×n).
However, the distance resolution was too low to differentiate 2 different ADP- and ATP-bound closed states, as was done with the Mn DEER. We cannot unambiguously assign the broader distance distribution to the open state. We note that in the case of DEER with spin labels, the spin-label flexibility adds to the distance distribution, thus compromising resolution. However, the excessive broadening observed for the proxyl spin label compared to the Gd results on the C-domain suggests that other spin-label effects may contribute to the width, as we discussed earlier. The broad distance distribution obtained with the proxyl spin labels is in line with a thesis from the Kay group reporting on DEER on MTSL-labeled yHsp90 mutants, where a large ensemble of conformational states that did not change upon nucleotide addition was also found. Cells couple the exergonic reaction of ATP hydrolysis with the endergonic reactions of cellular processes.
Epr Spectroscopy And Data Analysis
Adenosine triphosphate is an organic compound and hydrotrope that provides energy to drive many processes in living cells, such as muscle contraction, nerve impulse propagation, condensate dissolution, and chemical synthesis. Found in all known forms of life, ATP is often referred to as the “molecular unit of currency” of intracellular energy transfer. When consumed in metabolic processes, it converts either to adenosine diphosphate or to adenosine monophosphate . Other processes regenerate ATP so that the human body recycles its own body weight equivalent in ATP each day. The three phosphate groups are what enable ATP to store and release energy.
By donating free energy to the Na+/K+ pump, phosphorylation drives the endergonic reaction. Your metabolism is the collection of chemical reactions that occur in your cells to sustain life. Some of these reactions use stored energy to build things up, which we call anabolism, while other reactions break things down, releasing energy that can be stored for future use, and this is called catabolism. Imagine that the hamburger you’re having for dinner, made of proteins, fats, and carbohydrates, is a collection of lego blocks of various colors and shapes. It took a lot of energy to organize those blocks into that complex structure, and breaking the blocks apart releases that energy and frees the blocks so that they can be built back up into new things. The Adenosine triphosphate molecule is the nucleotide known in biochemistry as the “molecular currency” of intracellular energy transfer; that is, ATP is able to store and transport chemical energy within cells. ATP also plays an important role in the synthesis of nucleic acids.
The same was observed by SAXS data on AMP–PNP-bound HtpG, which was found to be in a mixture of open/closed conformations . Additionally, sm FRET data showed an open ⇄ closed equilibrium in the apo state, which was shifted toward the closed with AMP–PNP . In contrast, both open and closed conformations were detected with ATP under turnover conditions . Similar results were reported by another 3-color sm FRET study, where in the presence of ATP under turnover conditions or with ADP, the conformational transitions were found in thermal equilibrium and both nucleotides could bind to both open and closed conformations in yHsp90 .
Accelerated Master’s Degree Program
This displacement from equilibrium means that the hydrolysis of ATP in the cell releases a large amount of free energy. Specifically, we detected closed and compact conformations for the ATP-bound, prehydrolysis state and ADP-bound, posthydrolysis state, respectively. The conformation of the HES was found similar to that of the posthydrolysis state. This suggests that the presence of the inorganic phosphate prior to its release does not affect the inter–N-domain contacts in the ATPase region. Although the ATP-bound state has been extensively studied, little was known about the HES and ADP-bound states, which are highly relevant to Hsp90’s functional conformational cycle. It has been proposed that the ADP-bound state represents a second functional state upon which clients are displaced from the hydrophobic surface areas of Hsp90, allowing completion of the cycle. Our results, apart from probing a biologically important solution state of yHsp90, open the route to studying via EPR Hsp90 in the presence of cochaperones and clients to further elucidate the mechanism of action of this important protein.
Its absence suggests that under our conditions no transitions between open/closed conformations of similar probabilities of the C-domains occur. The W-band DEER results for the C-domain D560C/Gd and K637C/Gd mutants are given in Fig. The DEER traces revealed dipolar oscillations and distance distribution with maxima at ∼5.2 nm and ∼3.4 nm, respectively, for all hydrolysis states. The distance distributions are in good agreement with those predicted using the closed X-ray structure of yHsp90 (SI Appendix, Fig. S6) by the MtsslWizard software . The similar distance distributions for all hydrolysis states for the 2 mutants show that the C-domains are structured and dimerized, independent of the hydrolysis state, also in the absence of cochaperone binding. Hsp90 is an important, evolutionary conserved, molecular chaperone responsible for maintaining homeostasis of the cells upon heat and other stress conditions.
Because the bond in ATP is so easily broken and reformed, ATP is like a rechargeable battery that powers cellular process ranging from DNA replication to protein synthesis. The action in muscle cells provides an illustration of how ATP supplies energy to other molecules. Your muscles contract when one set of tiny molecules grip onto other molecules that are kind of like long cables in your muscle cells. When the pulling motion is finished, a gripping molecule has no ATP or ADP. A molecule of ATP fits onto the gripping molecule and immediately loses one phosphate group. The conversion from ATP to ADP transfers energy to the gripping molecule, which moves back to its grabbing position. It grabs onto the cable molecule and then relaxes back into its pulling position, where it gives up the ADP and gets ready for another ATP and the start of another gripping cycle.
What are 3 different coenzymes?
Coenzymes such as coenzyme A, acetyl coenzyme A, cellular redox coenzymes: NAD+ (oxidized nicotinamide adenine dinucleotide), NADH (reduced nicotinamide adenine dinucleotide), NADP+ (oxidized nicotinamide adenine dinucleotide phosphate) and NADPH (reduced nicotinamide adenine dinucleotide phosphate), energy coenzymes:
For example, transmembrane ion pumps in nerve cells use the energy from ATP to pump ions across the cell membrane and generate an action potential. The sodium-potassium pump (Na+/K+ pump) drives sodium out of the cell and potassium into the cell. When ATP is hydrolyzed, it transfers its gamma phosphate to the pump protein in a process called phosphorylation. The Na+/K+ pump gains the free energy and undergoes a conformational change, allowing it to release three Na+ to the outside of the cell. Two extracellular K+ ions bind to the protein, causing the protein to change shape again and discharge the phosphate.
The sample names indicated on the figure are summarized in SI Appendix, Table S1. Mn–Mn DEER time traces at different states of the ATP cycle in WT yHsp90.


