Atp And Adp

Content

The enzymes that participate in fatty acid catabolism are located in the mitochondria, along with the enzymes of the citric acid cycle, the electron transport chain, and oxidative phosphorylation. This localization of enzymes in the mitochondria is of the utmost importance because it facilitates efficient utilization of energy stored in fatty acids and other molecules. is a linear metabolic pathway of enzyme-catalyzed reactions that converts glucose into two molecules of pyruvate in the presence of oxygen or two molecules of lactate in the absence of oxygen.
What is PFK stimulated by?
Brain phosphofructokinase-1 is inhibited by ATP, Mg2+ and citrate and stimulated by NH4+, K+, PO43−, 5′-AMP, 3′,5′-cAMP, ADP and fructose-2,6-bisphosphate.
The ability to produce sufficient ATP by a pathway that does not require oxygen gives cancer cells a selective advantage over normal cells. Isolated mitochondria are analogous to engines without any fuel. Adding a fuel to a suspension of mitochondria in an appropriate medium sets the processes in motion, subject to the limitations imposed upon the mitochondria by lack of an intracellular environment. State IV respiration is defined as oxygen consumption by isolated mitochondria on a particular substrate, in the absence of ADP or any metabolic poisons or inhibitors. The energy in any mitochondrial substrate (succinate, glutamate, malate + pyruvate, fatty acids) is channeled to the ETS by a the reduced form of a ‘high energy intermediate,’ either NAD or FAD.
What Are The Four Phases Of Complete Glucose Breakdown?
Note the indication in the table that a variable amount of ATP is synthesized, depending on the tissue, from the NADH formed in the cytoplasm during glycolysis. This is because NADH is not transported into the inner mitochondrial membrane where the enzymes for the electron transport chain are located.
(For more information about oxidative phosphorylation, see Section 11.4 “Stage III of Catabolism”.) However, in the absence of oxygen , the fate of pyruvate is different in different organisms. In vertebrates, pyruvate is converted to lactate, while other organisms, such as yeast, convert pyruvate to ethanol and carbon dioxide. These possible fates of pyruvate are summarized in Figure 11.17 “Metabolic Fates of Pyruvate”. The conversion to lactate or ethanol under anaerobic conditions allows for the reoxidation of NADH to NAD+ in the absence of oxygen. When glucose enters a cell, it is immediately phosphorylated to form glucose 6-phosphate, in the first reaction of phase I.
Obligate ATP production via glycolysis also occurs in the absence of oxygen whether mitochondria are present or not. Overproduction of lactic acid by anaerobic glycolysis can lead to lactic acidosis, a life-threatening medical condition. Finally, even when both mitochondria and oxygen are present, cancer cells preferentially produce ATP by the conversion of glucose to lactate by aerobic glycolysis. The robust flux of glycolysis in cancer cells maintains high levels of intermediates required for the synthesis of macromolecules required for rapid growth and protection against reactive oxygen species. is a linear metabolic pathway of enzyme-catalyzed reactions that convert glucose into two molecules of pyruvate in the presence of oxygen or into two molecules of lactate in the absence of oxygen.
These reactions are referred to as a cycle because oxaloacetate is used in the first step and is regenerated in the last step. The citric acid cycle is analogous to a hand-cranked generator, where one turn of the crank produces energy in the form of electricity, while the crank itself is unaltered. That is, no new energy is generated, rather it is transformed from one form to another; therefore, the hand turning the crank provides the energy that will be converted to electricity. Using this analogy, the citric acid cycle is the generator, acetyl CoA provides the energy to turn the crank, and the energy of the carbon bonds are converted to the reduced electron carriers and ATP . The end-point of glycolysis is the formation of pyruvate , which can enter several different metabolic pathways depending on the type of organism and the presence of oxygen.
The presence of such a reaction in a catabolic pathway that is supposed to generate energy may surprise you. However, in addition to activating the glucose molecule, this initial reaction is essentially irreversible, an added benefit that keeps the overall process moving in the right direction. Furthermore, the addition of the negatively charged phosphate group prevents the intermediates formed in glycolysis from diffusing through the cell membrane, as neutral molecules such as glucose can do. Autophagy is a key evolutionarily conserved intracellular process that controls protein and organelle degradation and recycling.
However, in some cells, most notably mature red blood cells, glycolysis is the only means of ATP production because of the lack of mitochondria. In the absence of oxygen, glycolysis is the only option that cells have for the production of ATP from glucose. Overproduction of lactic acid by anaerobic glycolysis leads to lactic acidosis, a life-threatening condition. Many cancer cells have an exceptionally high enzymatic capacity for glycolysis. Even when oxygen is available, cancer cells produce much of their ATP by glycolysis.
Does facilitated diffusion require ATP?
A. Simple diffusion does not require energy: facilitated diffusion requires a source of ATP. Simple diffusion can only move material in the direction of a concentration gradient; facilitated diffusion moves materials with and against a concentration gradient.
In this tutorial we will focus on the first and last step, and the products of the citric acid cycle. Another factor that affects the yield of ATP molecules generated from glucose is the fact that intermediate compounds in these pathways are used for other purposes. Glucose catabolism connects with the pathways that build or break down all other biochemical compounds in cells, and the result is somewhat messier than the ideal situations described thus far. For example, sugars other than glucose are fed into the glycolytic pathway for energy extraction. Moreover, the five-carbon sugars that form nucleic acids are made from intermediates in glycolysis. Certain nonessential amino acids can be made from intermediates of both glycolysis and the citric acid cycle. Lipids, such as cholesterol and triglycerides, are also made from intermediates in these pathways, and both amino acids and triglycerides are broken down for energy through these pathways.
Steps In The Β
Transport of electrons and free energy proceeds, and the electrons plus some free eneregy are used by complex IV, the cytochrome oxidase complex, to reduce oxygen. Experimentally, the decline in total dissolved oxygen as oxygen is reduced to water can be used to measure the rate of electron transport. Reduction of oxygen produces water, but the significance of oxygen reduction is that it removes electrons from the system. Electrons must be removed so that the carriers can be re-oxidized and continue to transport and store free energy. The oxidation of fuel molecules , a process called respiration, is the source of energy used by cells. Catabolic reactions release energy from food molecules and use some of that energy for the synthesis of adenosine triphosphate ; anabolic reactions use the energy in ATP to create new compounds. In stage I, carbohydrates, lipids, and proteins are broken down into their individual monomer units—simple sugars, fatty acids, and amino acids, respectively.
This route lowers the yield of ATP to 1.5–2 molecules of ATP, rather than the usual 2.5–3 molecules. The amount of ATP formed by the oxidation of glucose depends on whether or not oxygen is present. Thus, approximately 42% of the energy released by the complete oxidation of glucose is conserved by the synthesis of ATP. In the absence of oxygen, only 2 molecules of ATP are formed for each molecule of glucose converted to lactate , and the amount of energy conserved is much less (2%). Like glucose, the fatty acids released in the digestion of triglycerides and other lipids are broken down in a series of sequential reactions accompanied by the gradual release of usable energy. Some of these reactions are oxidative and require nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide .
Contracting skeletal muscle supplies lactate to the liver, which uses it to synthesize glucose. Studies have shown that alanine, like lactate, is a major precursor of glucose. In muscle, alanine is formed from pyruvate by transamination (Section 24.2.2); the reverse reaction takes place in the liver.
Overall, in living systems, these pathways of glucose catabolism extract about 34 percent of the energy contained in glucose. Acetyl CoA is the entry point to the citric acid cycle, and while acetyl CoA will be oxidized and CO2released, this does not happen directly but occurs via an eight-step process. The first step of the citric acid cycle is the transfer of two carbons from acetyl CoA to the 4-carbon sugar oxaloacetate to generate the 6-carbon sugar citrate- hence, the name of the cycle. The end product of the citric acid cycle is oxaloacetate, which you should recall combines with acetyl CoA to start the cycle.
In the presence of oxygen, pyruvate enters the remaining stages of cellular respiration. Pyruvate is oxidized in a reaction that generates acetyl CoA, NADH and CO2 . Acetyl CoA is further oxidized to CO2 and H2O in the citric acid cycle . In stage II of catabolism, the metabolic pathway known as glycolysis converts glucose into two molecules of pyruvate (a three-carbon compound with three carbon atoms) with the corresponding production of adenosine triphosphate . The individual reactions in glycolysis were determined during the first part of the 20th century.
Pyruvate in the liver is converted into glucose by the gluconeogenic pathway. Glucose then enters the blood and is taken up by skeletal muscle. Thus, the liver furnishes glucose to contracting skeletal muscle, which derives ATP from the glycolytic conversion of glucose into lactate.
Glycolysis: Definition, Steps, Products & Reactants
It was the first metabolic pathway to be elucidated, in part because the participating enzymes are found in soluble form in the cell and are readily isolated and purified. The pathway is structured so that the product of one enzyme-catalyzed reaction becomes the substrate of the next. The transfer of intermediates from one enzyme to the next occurs by diffusion. The plasma membrane of most cells contains carriers that render them highly permeable to lactate and pyruvate. Both substances diffuse out of active skeletal muscle into the blood and are carried to the liver. Much more lactate than pyruvate is transported out because the high NADH/NAD+ ratio in contracting skeletal muscle favors the conversion of pyruvate into lactate. The lactate that enters the liver is oxidized to pyruvate, a reaction favored by the low NADH/NAD+ ratio in the cytosol of liver cells.

In stage II, these monomer units are broken down by specific metabolic pathways to form a common end product acetyl-coenzyme A . In stage III, acetyl-CoA is completely oxidized to form carbon dioxide and water, and ATP is produced. In the presence of oxygen, pyruvate is transformed into an acetyl group attached to a carrier molecule of coenzyme A. The resulting acetyl CoA can enter several pathways, but most often, the acetyl group is delivered to the citric acid cycle for further catabolism. During the conversion of pyruvate into the acetyl group, a molecule of carbon dioxide and two high-energy electrons are removed. The carbon dioxide accounts for two of the six carbons of the original glucose molecule. The electrons are picked up by NAD+, and the NADH carries the electrons to a later pathway for ATP production.
The latter pathway, anaerobic glycolysis, is believed to be the first process to have evolved in nature to produce adenosine triphosphate . In some cells—notably in mature red blood cells—glycolysis is the only means of ATP production because of the lack of mitochondria. In most cells glycolysis converts glucose to pyruvate which is subsequently oxidized to carbon dioxide and water by mitochondrial enzymes.
Biochemistry 08: The Citric Acid Cycle And The Electron Transport Chain
At this point, the glucose molecule that originally entered cellular respiration has been completely oxidized. Chemical potential energy stored within the glucose molecule has been transferred to electron carriers or has been used to synthesize a few ATPs. The citric acid cycle, which takes place in the mitochondria, is the third stage of cellular respiration and it completes the oxidation of glucose. Recall that in glycolysis, glucose is converted to two molecules of pyruvate, and then pyruvate is further oxidized to acetyl CoA. In the citric acid cycle, acetyl CoA is completely oxidized to CO2 and reduced electron carriers are generated in the form of NADH and another molecule, flavin adenine dinucleotide . In addition, ATP is generated through substrate-level phosphorylation.
The interplay between glycolysis and gluconeogenesis is summarized in Figure 16.34, which shows how these two pathways help to meet the energy needs of different cell types. The three steps listed above for glycolysis are regulated by allosteric regulation. Recall from the tutorial entitled Enzyme Kinetics and Catalysis, allosteric regulation of enzyme activity occurs due to a conformational change induced by the binding of both allosteric activators and inhibitors. Regulation of the rate of these three strongly exergonic reactions affects the overall rate of glycolysis. This mechanism balances the rate of glycolysis with the overall rate of cellular respiration and ATP synthesis.
The end products of the electron transport chain are water and ATP. A number of intermediate compounds of the citric acid cycle can be diverted into the anabolism of other biochemical molecules, such as nonessential amino acids, sugars, and lipids. These same molecules can serve as energy sources for the glucose pathways.
Steps Of Cellular Respiration
The final round of β-oxidation, once the chain has been shortened to four carbon atoms, forms two molecules of acetyl-CoA. β-oxidation also forms the reduced coenzymes FADH2 and NADH, whose reoxidation through the electron transport chain and oxidative phosphorylation leads to the synthesis of ATP. The efficiency of fatty acid oxidation in the human body is approximately 41%. The presence or absence of oxygen determines the fates of the pyruvate and the NADH produced in glycolysis.
- The level of free energy of the electrons drops from about 60 kcal/mol in NADH or 45 kcal/mol in FADH2 to about 0 kcal/mol in water.
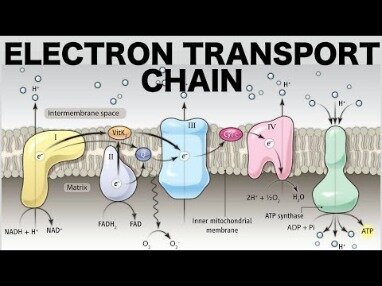
- The electron transport chain is composed of four large, multiprotein complexes embedded in the inner mitochondrial membrane and two small diffusible electron carriers shuttling electrons between them.
- The electron transport chain is the portion of aerobic respiration that uses free oxygen as the final electron acceptor of the electrons removed from the intermediate compounds in glucose catabolism.
- The electrons are passed through a series of redox reactions, with a small amount of free energy used at three points to transport hydrogen ions across a membrane.
- This process contributes to the gradient used in chemiosmosis.
Therefore, when cellular respiration is running well and the levels of intermediates (e.g. citrate and acetyl CoA) and ATP are high, the rate of glycolysis is reduced. Conversely, when citrate, acetyl CoA and ATP levels are low, the rate of glycolysis is increased. Fatty acids, released by the degradation of triglycerides and other lipids, are converted to fatty acyl-CoA, transported into the mitochondria, and oxidized by repeated cycling through a sequence of four reactions known as β-oxidation. In each round of β-oxidation, the fatty acyl-CoA is shortened by two carbon atoms as one molecule of acetyl-CoA is formed.
The electron transport chain is the portion of aerobic respiration that uses free oxygen as the final electron acceptor of the electrons removed from the intermediate compounds in glucose catabolism. The electron transport chain is composed of four large, multiprotein complexes embedded in the inner mitochondrial membrane and two small diffusible electron carriers shuttling electrons between them. The electrons are passed through a series of redox reactions, with a small amount of free energy used at three points to transport hydrogen ions across a membrane. This process contributes to the gradient used in chemiosmosis. The electrons passing through the electron transport chain gradually lose energy, High-energy electrons donated to the chain by either NADH or FADH2 complete the chain, as low-energy electrons reduce oxygen molecules and form water. The level of free energy of the electrons drops from about 60 kcal/mol in NADH or 45 kcal/mol in FADH2 to about 0 kcal/mol in water.
The phosphate donor in this reaction is ATP, and the enzyme—which requires magnesium ions for its activity—is hexokinase. In this reaction, ATP is being used rather than being synthesized.



