Energy, Atp, And Adp
Content
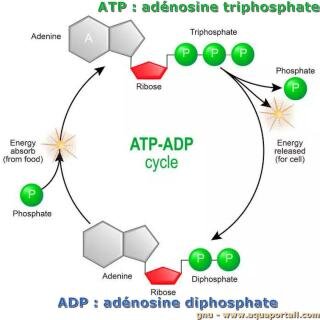
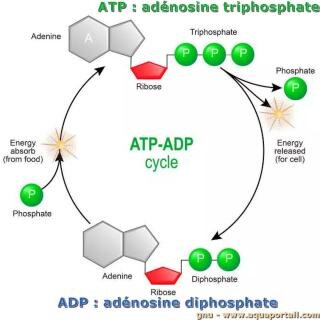
Adenosine triphosphate is comprised of the molecule adenosine bound to three phosphate groups. Adenosine is a nucleoside consisting of the nitrogenous base adenine and the five-carbon sugar ribose. The three phosphate groups, in order of closest to furthest from the ribose sugar, are labeled alpha, beta, and gamma. Together, these chemical groups constitute an energy powerhouse. The two bonds between the phosphates are equal high-energy bonds that, when broken, release sufficient energy to power a variety of cellular reactions and processes.
This leaves ADP and a free phosphate left over. During cellular respiration, the cell is able to reattach a phosphate onto the ADP molecule, making new ATP. Though many explanations are offered for the fatigue process in contracting skeletal muscle , none completely explain the decline in force production capability because fatigue is specific to the activity being performed. However, one needs to look no further than the muscle contraction crossbridge cycle itself in order to explain a major contributor to the fatigue process in exercise of any duration.
The more bonds in a molecule, the more potential energy it contains. Because the bond in ATP is so easily broken and reformed, ATP is like a rechargeable battery that powers cellular process ranging from DNA replication to protein synthesis.
What type of macromolecule is ATP ADP?
ATP & ADP are nucleic acids. Explanation: ATP and ADP are macromolecules of nucleic acids as they are made up of structures similar to DNA. An ADP molecule is made up of two phosphate groups, a sugar backbone (ribose) and the nucleotide adenine.
Unless quickly used to perform work, ATP spontaneously dissociates into ADP + Pi, and the free energy released during this process is lost as heat. To harness the energy within the bonds of ATP, cells use a strategy called energy coupling. The energy released from the hydrolysis of ATP into ADP is used to perform cellular work, usually by coupling the exergonic reaction of ATP hydrolysis with endergonic reactions. ADP can be interconverted to adenosine triphosphate and adenosine monophosphate . ATP contains one more phosphate group than does ADP. Energy transfer used by all living things is a result of dephosphorylation of ATP by enzymes known as ATPases. The cleavage of a phosphate group from ATP results in the coupling of energy to metabolic reactions and a by-product of ADP.
The energy released by the electrical potential across the membrane causes an enzyme, known as ATP synthase, to become attached to ADP. ATP synthase is a huge molecular complex and its function is to catalyze the addition of a third phosphorous group to form ATP. A single ATP synthase complex can generate over 100 molecules of ATP each second. Adenosine diphosphate and adenosine triphosphate are organic molecules, known as nucleotides, found in all plant and animal cells. ADP is converted to ATP for the storing of energy by the addition of a high-energy phosphate group. The conversion takes place in the substance between the cell membrane and the nucleus, known as the cytoplasm, or in special energy-producing structures called mitochondria. Examples are the splitting of ATP in muscle contraction and the transport of ions across cell membranes.
What Are The Functions Of Photosynthesis?
The bond between the beta and gamma phosphate is considered “high-energy” because when the bond breaks, the products [adenosine diphosphate and one inorganic phosphate group ] have a lower free energy than the reactants . ATP breakdown into ADP and Pi is called hydrolysis because it consumes a water molecule (hydro-, meaning “water”, and lysis, meaning “separation”). Now, what would happen if you took your quarter, and added to a group of three other quarters?
What happens when there is too much ATP in a cell?
When the amount of ATP is available in excess of the body’s requirements, the liver uses the excess ATP and excess glucose to produce molecules called glycogen. Glycogen is a polymeric form of glucose and is stored in the liver and skeletal muscle cells.
As you can see in this diagram, the oxygen on the bottom has a negative charge. Negative charges repel one another, and that makes breaking the bonds between the phosphates relatively easy for enzymes to do. In other words, it only takes a small amount of energy to break the bond that holds the third phosphate in ATP onto the rest of the molecule. When that phosphate is placed onto another molecule, energy is release for cellular work. It’s how your nerve cells set themselves up to send impulses, which allows you to do the thinking that you’re doing now. The hydrolysis of ATP is highly exergonic and used to drive numerous endergonic biochemical processes.ATP acts as a free-energy donor in most energy-requiring processes such as motion, active transport, or biosynthesis. • ATP transfer energy from the breakdown of food molecules to cell function.
Atp Adp Cycle
Breaking one of ATP’s phosphorus bonds generates approximately 30.5 kilojoules per mole of ATP (7.3 kcal). ADP can be converted, or powered back to ATP through the process of releasing the chemical energy available in food; in humans, this is constantly performed via aerobic respiration in the mitochondria. Plants use photosynthetic pathways to convert and store energy from sunlight, also conversion of ADP to ATP. Animals use the energy released in the breakdown of glucose and other molecules to convert ADP to ATP, which can then be used to fuel necessary growth and cell maintenance. adenosine triphosphate a nucleotide, adenosine 5′-triphosphate, occurring in all cells, where it stores energy in the form of high-energy phosphate bonds.
ATP is continually reformed from lower-energy species ADP and AMP. The biosynthesis of ATP is achieved throughout processes such as substrate-level phosphorylation, oxidative phosphorylation, and photophosphorylation, all of which facilitate the addition of a phosphate group to ADP. Your metabolism is the collection of chemical reactions that occur in your cells to sustain life.
Living things break down the three major categories of foods into their constituent parts, the individual lego blocks, for two reasons. The cell has a special kind of molecule for storing that energy, and it’s called ATP. Adenosine diphosphate and adenosine triphosphate both play important roles in providing cellular energy. When the cell needs to perform work, it removes a phosphate from ATP, releasing energy.
Atp Model Activity
ATP is also used to produce high-energy phosphorylated intermediary metabolites, such as glucose 6-phosphate. During cellular metabolic reactions, or the synthesis and breakdown of nutrients, certain molecules must be altered slightly in their conformation to become substrates for the next step in the reaction series. In the very first steps of cellular respiration, glucose is broken down through the process of glycolysis. ATP is required for the phosphorylation of glucose, creating a high-energy but unstable intermediate. This phosphorylation reaction causes a conformational change that allows enzymes to convert the phosphorylated glucose molecule to the phosphorylated sugar fructose. Fructose is a necessary intermediate for glycolysis to move forward. In this example, the exergonic reaction of ATP hydrolysis is coupled with the endergonic reaction of converting glucose for use in the metabolic pathway.
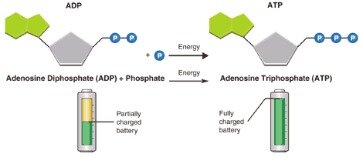
• Energy is released when phosphate group is removed • ADP is charge into ATP when phosphate group is added. Living cells use ATP as if it were power from a rechargeable battery. Converting ADP to ATP adds power, while almost all other cellular processes involve the breakdown of ATP and tend to discharge power.
It will again leave behind ADP and a phosphate. This recycling of ADP into ATP, and ATP breakdown into ADP, is called the ATP/ADP cycle. Cells couple the exergonic reaction of ATP hydrolysis with the endergonic reactions of cellular processes. For example, transmembrane ion pumps in nerve cells use the energy from ATP to pump ions across the cell membrane and generate an action potential. The sodium-potassium pump (Na+/K+ pump) drives sodium out of the cell and potassium into the cell. When ATP is hydrolyzed, it transfers its gamma phosphate to the pump protein in a process called phosphorylation. The Na+/K+ pump gains the free energy and undergoes a conformational change, allowing it to release three Na+ to the outside of the cell.
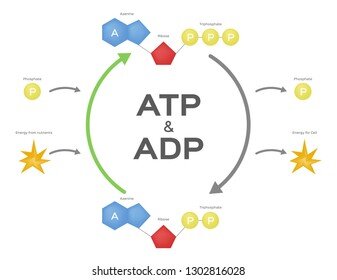
In the human body, a typical ATP molecule enters the mitochondria for recharging as ADP thousands of times a day, such that the concentration of ATP in a typical cell is about 10 times higher than that of ADP. Skeletal muscles require large amounts of energy for mechanical work, so muscle cells contain more mitochondria than the cells of other tissue types.
Two extracellular K+ ions bind to the protein, causing the protein to change shape again and discharge the phosphate. By donating free energy to the Na+/K+ pump, phosphorylation drives the endergonic reaction. Adenosine triphosphate is the energy currency for cellular processes. ATP provides the energy for both energy-consuming endergonic reactions and energy-releasing exergonic reactions, which require a small input of activation energy. When the chemical bonds within ATP are broken, energy is released and can be harnessed for cellular work.
- Adenosine triphosphate is comprised of the molecule adenosine bound to three phosphate groups.
- The two bonds between the phosphates are equal high-energy bonds that, when broken, release sufficient energy to power a variety of cellular reactions and processes.
- The three phosphate groups, in order of closest to furthest from the ribose sugar, are labeled alpha, beta, and gamma.
- Adenosine is a nucleoside consisting of the nitrogenous base adenine and the five-carbon sugar ribose.
- Together, these chemical groups constitute an energy powerhouse.
Your cells are able to recombine pieces to make new ATP molecules. During a process called cellular respiration, the cell uses energy from food, including sugar, proteins, and fats, and connects a free phosphate molecule onto an ADP molecule, creating ATP. The ATP will then be used for more cellular work.
The combined effects of these accumulated hydrolysis byproducts accounts for a large amount of the fatigue process in exercise of any intensity or duration. The three phosphate groups are what enable ATP to store and release energy. Each phosphate group consists of one phosphorus atom surrounded by oxygen atoms.
Pi release is coupled to the powerstroke of the crossbridge cycle. The accumulation of Pi during exercise would lead to a reversal of its release step, therefore causing a decrement in force production capability. Due to the release of Pi with both the immediate energy system and the hydrolysis of ATP, Pi accumulation is probably the largest contributor to the fatigue process in exercise of any duration. ADP release occurs near the end of the crossbridge cycle and therefore controls the velocity of crossbridge detachment. Therefore, ADP accumulation, which occurs during exercise of extended duration , causes a slowing of the rate constants . in the crossbridge cycle and a reduced oscillatory power output.



